CAR-T CELL THERAPY (part2)
Chimeric antigen receptors
Genetic modification of T cells with CARs represents a breakthrough for genetic engineering in hematological malignancies. The initial concept of CAR is based on cloning the TCR CD3 chain, which can independently activate T cells. First-generation CARs are created from variable-heavy and variable light sequences of a monoclonal antibody (mAb) specific to a tumor cell surface molecule and a cytoplasmic CD3 chain signaling field and contain only a single-chain variable fragment (scFv). Initial studies were conducted in patients with HIV infection with a longer survival. In first-generation cancer studies, CAR T cells did not multiply in vivo and persistence did not last long, or T cells were present at very low frequencies. Based on a second genetic modification, CARs have an antibody-based extracellular receptor structure that binds to target cells and intracellular activating areas. In second- and third-generation CARs, costimulatory protein receptors (e.g., CD28, CD137 (4-1BB), ICOS, CD134 (OX40), CD27, or CD244) were added to the cytoplasmic tail of the CAR (Figure 2.2.1). Second-generation BABs are built with one co-stimulant molecule, while third-generation BABs contain multiple additional co-stimulant molecules. The antitumor activity of CAR-T cells varies due to differences in the cytoplasmic field and the ability of the extracellular area to recognize a different epitope of the same antigen with different affinities for each CAR structure. As with third-generation CARs, the question of whether the addition of secondary cost incentives provides greater efficacy remains unanswered. CARs have several advantages: initiation of reliable high-power signals, HLA independence, no need for antigen processing, and no competition for CD3. The number of targets on tumor cells binding to CARs is greater than the number of major histocompatibility complex (MHC)/peptide complexes, and scFv has a higher binding affinity for antigens than TCRs. He suggested that when comparing the functional characteristics of genetically engineered T cells that express natural low-affinity αβ-TCR chains with high-affinity TCR-like Ab-based CARs targeting the same specificity, the upper affinity threshold should be used to mediate genetically effective functional outcomes. designed T cells. The major disadvantage of CARs is the large release of cytokines, which is triggered by the binding and immunogenicity of the mouse-derived scFv section of the CAR complex, which can lead to immune responses and the elimination of CAR T cells. In addition, intracellular molecules cannot be recognized [13].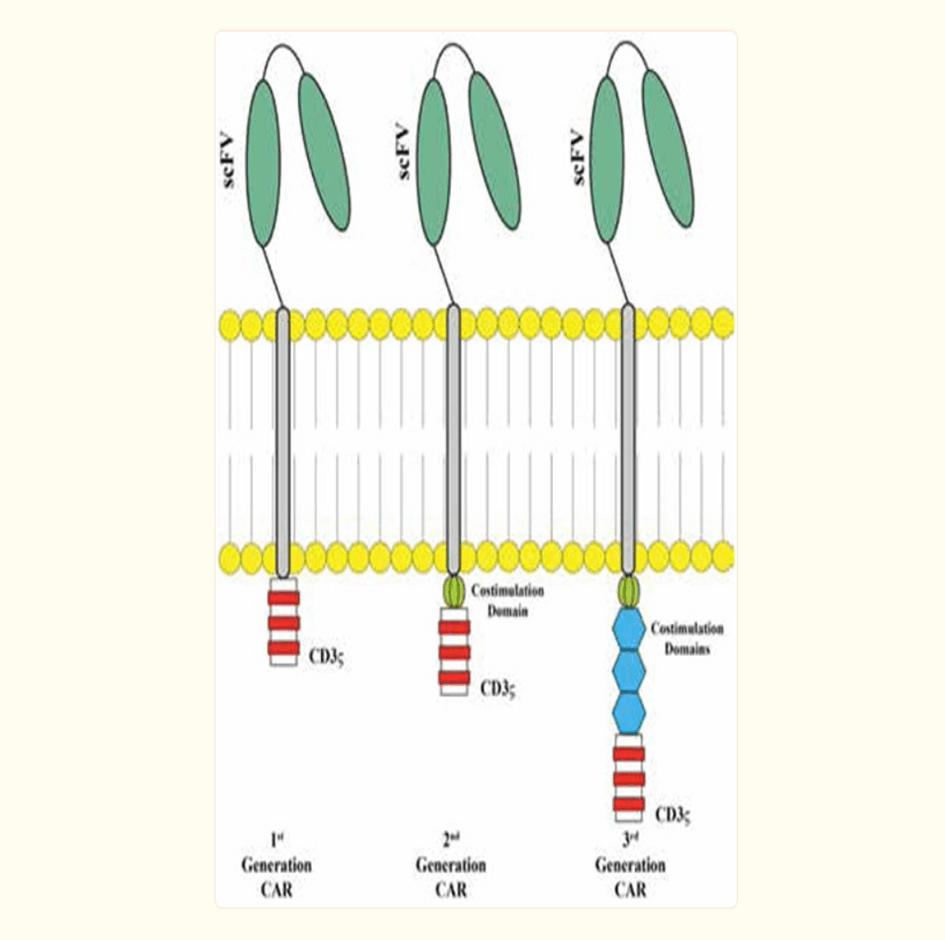
Chimeric antigen receptor T cell manufacturing
Gene transfer technology has evolved rapidly; however, clinical production of therapeutic CARs is limited to specialized, licensed manufacturing facilities with strict rules. In vitro culture systems for T cell expansion are used to generate large quantities of genetically engineered T cells. The average production time to generate the large numbers of unselected CD4 and CD8 T cells required for treatment is 10-14 days in clean rooms. First, mononuclear cells are isolated from the patient's peripheral blood by leukapheresis and T cells are selected with paramagnetic anti-CD3/anti-CD28 beads. Recent studies have shown that less differentiated T cells have a higher transplantation rate and antitumor activity. In particular, central memory CD8 T cells can be replaced by tumor-specific CARs. T cells are then transformed with a CAR-encoding viral vector. Two vector systems, retroviral or lentiviral vectors, can be used to deliver CAR-encoding genes into T cells. Retroviral vectors have persistent gene expression; however, transduction can only be performed on efficiently dividing T cells. Lentiviral vectors can also be integrated into non-dividing cells. The disadvantages of viral vectors are the cost and the expertise required to produce them. Transposon systems such as Sleeping Beauty 100X (SB100X) or PiggyBac (PB) are new methods for genetic modification of T cells with high gene expression; they are simple and inexpensive, and have a large loading capacity and low immunogenicity. T cells are grown in culture by stimulation with the anti-CD3 clone OKT3 with cytokines such as IL-2, IL-7 and IL-15. Additionally, in vivo persistence can be achieved by overexpression of anti-apoptotic proteins. CARs on T cells bind to their antigens on the tumor, and activation is controlled by intracytoplasmic domains within the CAR. Tumor killing may be mediated by direct cytotoxicity of CD8+ CAR T cells with granzyme and perforin or by cytokines released by CD4+ CAR T cells that bypass MHC. Long-term eradication and prevention can be achieved with memory T cells CAR from a single infusion [11,14].
2.4 Chimeric antigen receptor structure
CARs consist of four regions: an antigen recognition domain, an extracellular hinge region, a transmembrane domain, and an intracellular T-cell signaling domain [15].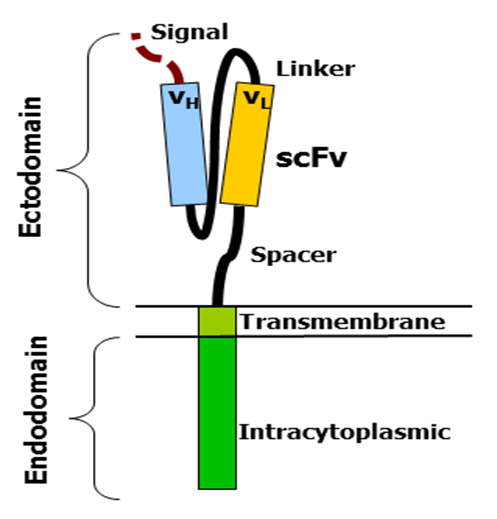
2.4.1 Antigen recognition domain
The antigen-recognition domain is exposed outside the cell at the ectodomain portion of the receptor. interacts with possible target molecules and is responsible for targeting the CAR-T cell to any cell. The antigen recognition domain is typically derived from the variable regions of a monoclonal antibody linked together as a single chain variable fragment (scFv). An scFv is a chimeric protein composed of light (VL) and heavy (VH) chains of immunoglobins linked by a short linker peptide. These VL and VH regions are preset according to the target antigen [15,16].
2.4.2 Hinge area
The hinge, also called the spacer, is a small structural space located between the antigen-recognition site and the outer membrane of the cell. The optimal hinge increases the flexibility of the scFv receptor head and reduces spatial constraints between the CAR and its target antigen. This promotes antigen binding and synapse formation between CAR-T cells and target cells. Hinge arrays are often based on membrane proximal regions of other immune molecules, including IgG , CD8 and CD28 [15,17].
2.4.3 Transmembrane domain
The transmembrane domain spans the cell membrane with the hydrophobic alpha helix. It anchors the CAR to the plasma membrane and bridges the antigen-recognition domain to the intracellular signaling domain. This domain is essential for the stability of the receptor as a whole [17].
2.4.4 Intracellular T cell signal domain
The intracellular T cell signaling domain is located inside the cell, at the enddomain of the receptor. After an antigen binds to the external antigen recognition site, the CAR receptors aggregate and transmit an activation signal. The internal cytoplasmic end of the receptor then resumes signaling inside the T cell [9,18].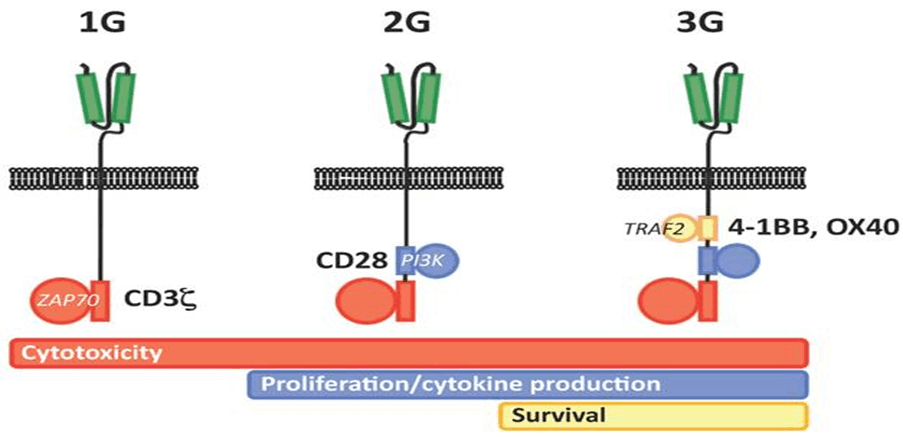





























































![Nekodex – Earn 20K+ NekoCoin ($20) [Highly Suggested]](https://cdn.bulbapp.io/frontend/images/b4f0a940-f27c-4168-8aaf-42f2974a82f0/1)


