Unveiling the Mysteries: A Comprehensive History of the Atom
Introduction:
The atom, the fundamental building block of matter, has a rich and intriguing history that spans centuries. The journey of understanding its nature has been a collaborative effort, with contributions from brilliant minds across different cultures and eras. In this blog, we will embark on a fascinating journey through the history of the atom, from ancient Greek philosophy to modern quantum mechanics.
Ancient Greek Philosophy:
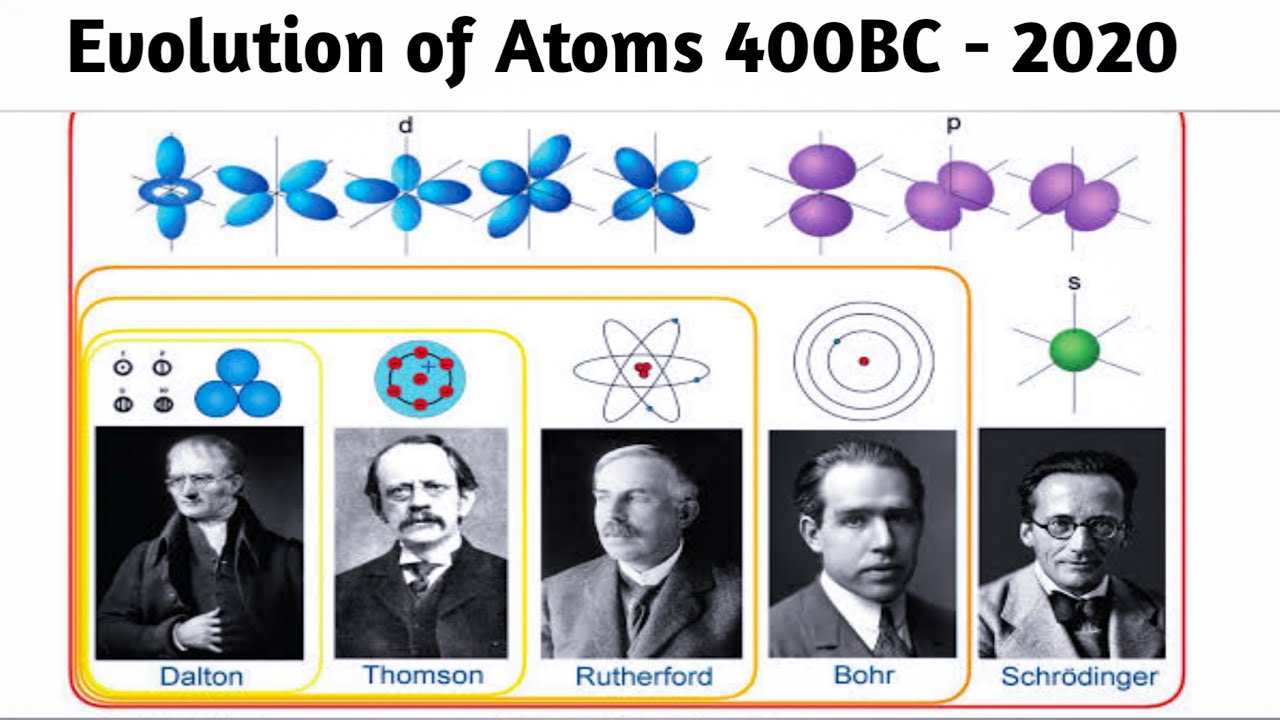
The concept of the atom can be traced back to ancient Greek philosophers. Around 400 BCE, the renowned philosopher Democritus proposed the idea that matter consists of indivisible particles called "atoms," derived from the Greek word "atomos," meaning uncuttable. Democritus envisioned these atoms as tiny, indestructible entities that combine in various ways to form different substances.
However, the atomic model proposed by Democritus lacked experimental evidence, and his ideas were overshadowed by Aristotle's influential views on matter. It wasn't until much later that the concept of atoms regained attention.
Dalton's Atomic Theory:
In the early 19th century, John Dalton revived the atomic theory, providing a more scientific foundation. In 1803, Dalton proposed a set of postulates that laid the groundwork for understanding the behavior of matter at the atomic level. His theory suggested that elements are composed of indivisible atoms, each with a specific mass, and that chemical reactions involve the rearrangement of these atoms.
Dalton's work paved the way for a more systematic exploration of the atom, but it was still a relatively simplistic model that needed refinement.
Thomson's Discovery of the Electron:
In the late 19th century, experiments with cathode rays led to a breakthrough by J.J. Thomson. In 1897, he discovered the electron, a subatomic particle with a negative charge. This discovery challenged the idea of indivisible atoms, introducing the concept that atoms could be divided into smaller components.
Thomson proposed the "plum pudding" model, envisioning electrons embedded in a positively charged "pudding" like raisins in a cake. This marked a significant advancement in our understanding of atomic structure.
Rutherford's Nuclear Model:
Ernest Rutherford further advanced atomic theory through his famous gold foil experiment in 1909. His findings suggested that atoms have a small, dense nucleus at their center, containing positively charged protons. The majority of the atom's volume is empty space, with electrons orbiting the nucleus.
Bohr's Quantum Model:
In 1913, Niels Bohr refined the model by introducing the concept of quantized electron orbits. Bohr's model successfully explained the spectral lines of hydrogen and provided a more accurate description of atomic structure. However, it had limitations, particularly for larger atoms.
The Quantum Mechanical Model:
The advent of quantum mechanics in the early 20th century revolutionized our understanding of the atom. Scientists like Werner Heisenberg and Erwin Schrödinger developed the quantum mechanical model, which describes the behavior of electrons as probability distributions rather than fixed orbits.
Modern advancements in quantum mechanics, including the development of quantum field theory, have provided a more intricate understanding of subatomic particles, such as quarks and leptons.
Conclusion:
The history of the atom is a testament to human curiosity, perseverance, and collaboration. From ancient philosophical ponderings to the intricate theories of modern quantum mechanics, the journey has been marked by groundbreaking discoveries and paradigm shifts. Today, our understanding of the atom continues to evolve as scientists delve deeper into the mysteries of the subatomic world, unlocking new realms of knowledge and pushing the boundaries of scientific exploration.