Purification of Proteins
Purification of Proteins
AIM : In this experiment, our aim purify the blood protein hemoglobin as an example of protein purification methods.
INTRODUCTION :
Several thousand proteins have been purified in active form on the basis of such characteristics as solubility, size, charge, and specific binding affinity. Usually, protein mixtures are subjected to a series of separations, each based on a different property to yield a pure protein.
Most proteins are less soluble at high salt concentrations, an effect called salting out. The salt concentration at which a protein precipitates differs from one protein to another. (1)
Centrifugation is a technique that provides separation according to the behavior of the particles in an area where relative centrifugal force is generated by rotating at high speed with the help of centrifuges.(2) 
Affinity Chromatography is a separation technique based upon molecular conformation, which frequently utilizes application specific resins. These resins have ligands attached to their surfaces which are specific for the compounds to be separated. (4)
MATERIALS : -Blood sample with anticoagulant -0.9% NaCl -Distilled water -Toluene
-Ependorf tube -Micropipette -Pipette -EDTA -Centrifugation machine
METHOD:
Firstly we take 1 ml blood samples with EDTA.
We put 1 ml of blood into the eppendorf tubes with a micropipette. We placed our tubes mutually balanced way in centrifugation.
We centrifuged twice to see the two phases. Firstly centrifugation for 5 min at 10.000 rpm. After centrifugation for 5 min at 14.800 rpm(highest rpm), the upper plasma portion is discarded with a pipette.
The blood sample is washed three times with saline(NaCl) as the ratio of 1:1. We took the supernatant part with a micropipette.
Washing Process: 1ml NaCl is added to the sample and shaken gently. Then, centrifugation for 5 min at 3000 rpm. We did it 3 times. Discard the supernatant.
After centrifugation, distilled water as 1.5 times of the underlying layer of red blood cells (~750 μl) and toluene as half volume of red blood cells (~250 μl) are added. Shake with hands for 8 min.
Centrifugation for 20 min at 3000 rpm. The supernatant is taken carefully with micropipette.
We put our samples 4⁰C for use in our next experiment.
RESULT: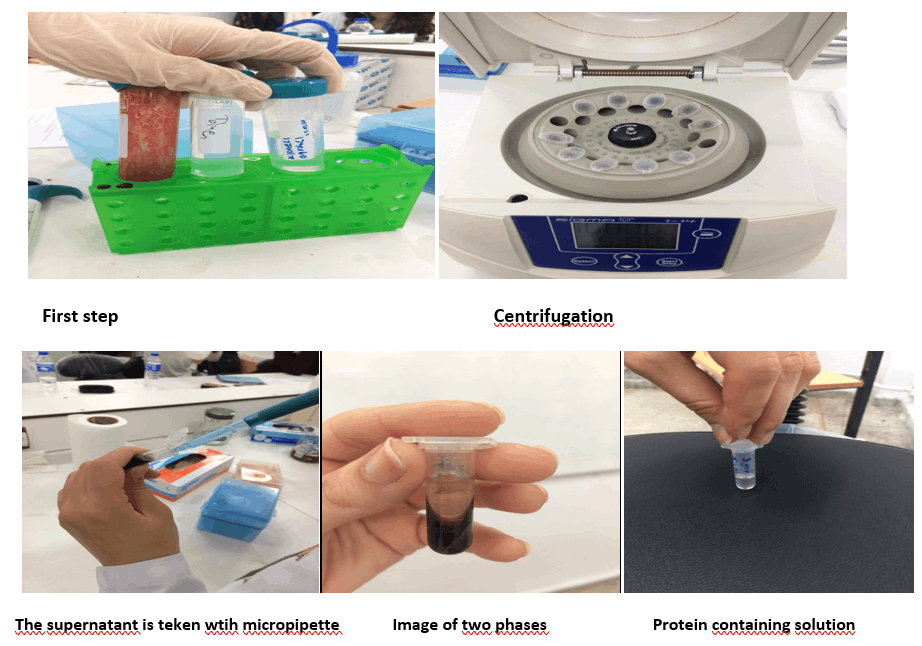
EDTA: It prevents blood clotting by binding Ca++ ions. (anticoagulant)
Retic Saline: In washing process, it is used to remove any residue left from plasma formed by the agglomeration of red blood cells. (Retic Saline: NaCl, KCl and MgCl2)
We used %0.9 NaCl as saline in our experiment.
Toluene: It causes the disintegration of the cell membrane by dissolving the lipid bilayer of the cell membrane disintegration of the cell membrane.
Purification steps;
-Extraction
-Purification of Proteins: Water removal , Removal of salt and small ions , Separation from other proteins
-Primary structure determination: The type, number and order of amino acids
In the first step of our experiment, we should put it in the centrifuge once, but we put it twice. The reason for this was that we wanted to see both phases.
After centrifugation, we pulled the supernatant portion with a micropipette because we wanted to remove structures that were not required( vitamins, organelles etc.) in our experiment.
Affinity chromatography makes a purer separation. Separation of ions that are specific to each other.
Two-dimensional polyacrylamide gel electrophoresis is better. It distinguishes in terms of acidity, basicity, pH and size. If gel electrophoresis and isoelectric focusing are used together, purification is achieved as two-dimensional polyacrylamide gel electrophoresis but slower. That's why it is more profitable to use two-dimensional polyacrylamide gel electrophoresis for us.
REFERENCES:
(1) https://www.ncbi.nlm.nih.gov/books/NBK22410/
(2) http://bys.trakya.edu.tr/file/open/78380515
(3) https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/General_Information/1/ge-strategies-for-protein-purification.pdf
http://www.biologydiscussion.com/biochemistry/protein-purification/methods-of-protein-purification-4-methods/12962












































































